Agentic AI for MedTech quality & launches
Accelerate healthcare
innovation & quality.
Compliance, product development and real world operational scaling are disconnected. Qyro turns standards into a living system that stays current as you build and scale.
For MedTech & providers
Device, SaMD and digital health teams; NHS and private providers.
Lifecycle coverage
From first document trail to go-live, change control and live assurance.
Platform + leadership
Agentic AI backed by CSOs, DPOs and fractional quality leadership.
How Qyro works
Success simplified. Joined up compliance.
We combine an agentic AI platform with specialist leadership so you can understand what's required, build the right system and keep it working as you ship and scale.
Understand your landscape
Qyro onboards your organisation, products and deployments, mapping them to the relevant standards and obligations for suppliers and providers.

Build a living quality system
Generate, tailor and manage a structured library of documents, hazards and training, all mapped clause-by-clause to the standards that matter.

Operate with continuous assurance
Keep your posture current as you ship, iterate and scale. Qyro tracks evidence, changes and reviews so audits feel calm, predictable and organic.

Inside the Qyro workspace
A single home for documents, risks, training and evidence.
Qyro gives your teams one calm, structured environment to manage their quality work: tailored document libraries, hazard and risk logs, guided checklists and training all linked back to the standards you care about.
Clause-level traceability from documents and tasks back to ISO, DCB, DTAC, DSPT and more.
Rich views across products, deployments and owners so nothing gets lost between teams.
Built-in tasks, logs and training modules so quality becomes part of the day-to-day workflow.
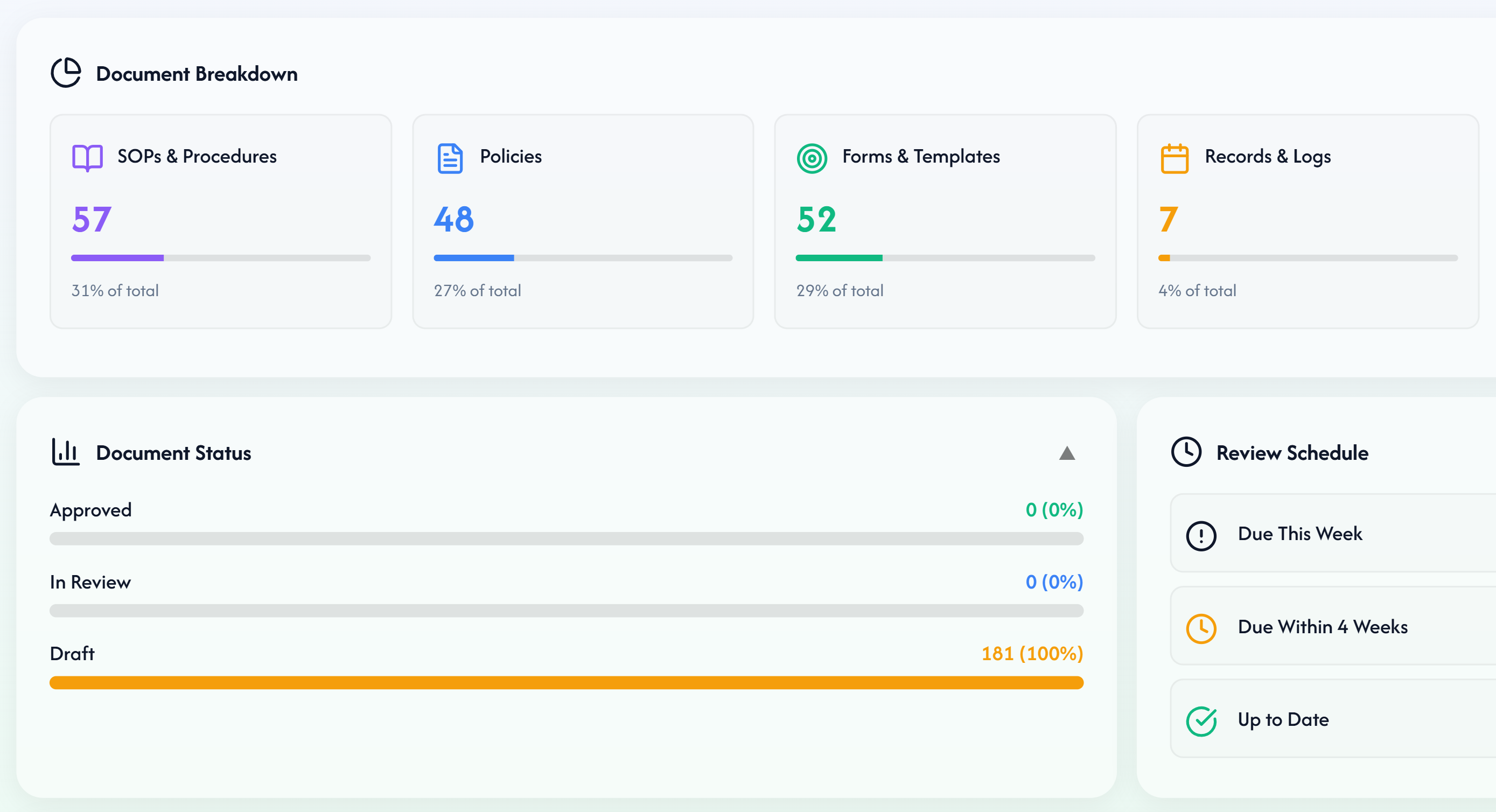
How we can support
Product, compliance and assurance support for MedTech suppliers and providers.
Combine Qyro's agentic AI platform with specialist advisory and leadership services. Whether you're a supplier deploying into health systems or a provider assuring complex supplier landscapes, we help you move faster while staying safe and compliant.
Standards we help you meet














Qyro supports you across the full lifecycle — from first deployment through change control to ongoing assurance against these standards.
Why teams choose Qyro
Tools designed to drive quality.
Qyro brings together agentic regulatory intelligence, execution tooling and specialist leadership — enabling MedTech teams to move faster without compromising safety or assurance.

How Qyro feels in use
One space to understand, act and stay ahead.
From the first document to portfolio-level oversight, Qyro shows teams exactly what’s needed and what happens next — keeping quality predictable and calm.
Agentic regulatory intelligence
Qyro interprets ISO and other major standards and frameworks in context — tailoring requirements to your product, evidence and lifecycle. Not generic checklists or templates.
Total lifecycle visibility
One connected view across strategy, hazards, risks, evidence, documents and releases — enabling leadership to see compliance posture instantly and focus investment where it matters.
Execution built in
AI-generated documents, flowcharts, logs and checklists; guided approvals, training, and versioning. Everything teams need to embed quality and ship safely — not just assess it.
Continuous assurance
Qyro actively monitors change, risk and evidence across your portfolio. Real-time insight replaces point-in-time audits, lowering the cost and burden of compliance.
Amplified by expert leadership
Clinical safety officers, regulatory advisors, fractional leaders and technical assurance services combine with the platform to provide end-to-end confidence, not just software.
Regulator-ready from day one
Built for the scrutiny of notified bodies, NHS assurance teams and clinical safety officers — with verifiable audit trails, controlled evidence chains and rapid, low-friction deployment.
Product & service tiers
Right-sized support for where you are now.
Each tier combines the Qyro platform with advisory and leadership support. We shape the engagement around your products, markets and regulatory obligations.
Get organised quickly with core workflows, essential documents and guided setup.
Add automation, portfolio views and coverage across multiple standards and stakeholders.
Support multi-entity governance, deeper assurance and leadership engagement.
See Qyro in action
Watch the platform bring your quality system to life.
In a live walkthrough we'll show how Qyro turns standards into a living system – generating tailored documents, surfacing risks and embedding training, all in a single, calm workspace.
See how Qyro builds and maintains your tailored document library and audit trail.
Explore risk logs, clinical safety and IG posture in one aligned view.
Walk through targeted training and how teams evidence understanding.




